Docusign IAM | Life Sciences Modules for 21 CFR Part 11
Spend time on patients, not paperwork
Improve the patient, physician and employee experience

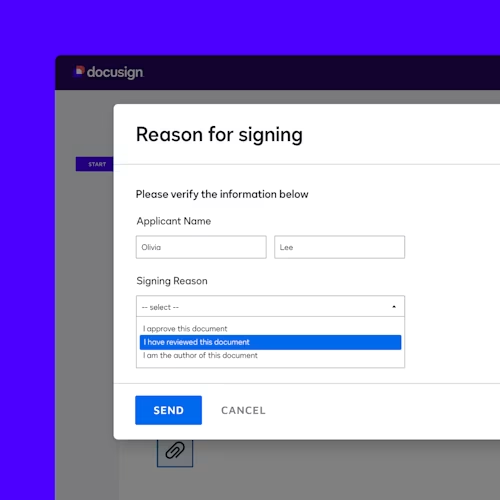
Docusign offers modules to support your compliance with the electronic signature practices set forth in the U.S. Food and Drug Administration’s 21 CFR Part 11. Our core Part 11 module includes Part 11-specific eSignature functionality for authentication, reason for signature and signature manifestation. These capabilities help you comply with regulations while using eSignature to make executing agreements faster, more cost-efficient and more convenient for everyone involved.
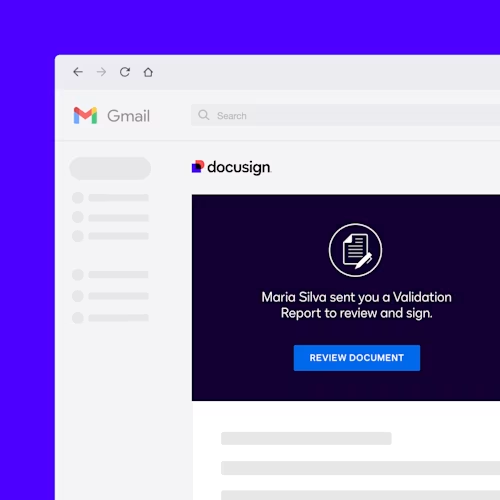
An additional module is the Docusign Validator for Life Sciences, which simplifies your validation testing and documentation required for Part 11 compliance. Its reports contain screenshots of each test, details of the specific provision tested, and the final results.
Account-wide controls
Apply module settings to all envelopes sent from your account to enable consistent compliance.
Simplify compliance validation
Receive automated reports regarding Part 11 functionality compliance.
Authenticate two ways
Require two-factor authentication to access and sign documents.
Know who did what
View a detailed audit trail and Certificate of Completion for each transaction.
FAQ
Life sciences organizations use Docusign to reduce time to market, simplify global collaboration, and improve the patient, physician, and employee experience.
See the white paper Using Docusign to Facilitate Compliance with 21 CFR Part 11.
See the support page for the Validator.
If you want to do it yourself, the Docusign Validator for Life Sciences will help. If you’d like to engage a third party, our partner USDM provides technology and services that enable life science customers to achieve and maintain Part 11 compliance.